by Dr M Carmo Saunders-Vasconcelos, Eastern Institute of Technology, Napier, New Zealand
Sustainable irrigation practices require that water is used strictly when necessary to maximize water use efficiency, avoid waste and prevent contamination of groundwater with leachates. Water is increasingly scarce and costly and the water footprint has become the measure of sustainability. Unrestricted water supply is no longer an option and the Hawke’s Bay wine industry needs to prepare for the challenging future. The economic risk associated with wine grape production will increase and strategies to minimize loss of production while maintaining fruit and wine quality need to be developed.
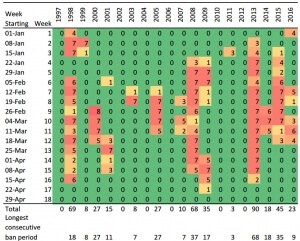
Irrigation consents in many regional councils across New Zealand are now linked to increasingly higher low river flow limits, with decision makers shifting their emphasis from production values to environmental and recreational values (Harding 2016). As an example, the low flow limit for the Ngaruroro river, affecting the Gimblett gravels and large areas of Bridge Pa, Maraekakaho, Mangatahi, and Crownthorpe, is subject to a Water Conservation Order application to raise from 2,400L/s to 4,200L/s. Tables 1 and 2 show the number of irrigation ban days in the past 20 years that these two low flow limits would trigger. The period selected encompassing January 1 to April 30 is the one when river flows may be lower than stipulated limits. Only 3 days of flows lower than 4,200 L/s occurred in December in the 20-year period (two days in 2007 and one day in 2015).
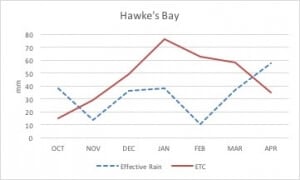
Changing the low flow limit will have a dramatic impact on the frequency of long ban periods. In Hawke’s Bay, premium vineyards are located in free draining gravelly soils. Figure 1 shows vineyard water requirements (crop evapotranspiration) and precipitation. It is evident that during the hottest summer months, there is a deficit between transpiration water and amount of precipitation, which needs to be supplemented by irrigation. Failing to do so, will result in reduced yields of unacceptable quality and will compromise plant survival.
Table 1. Number of days per week in the period of January 1 to April 30 when the Ngaruroro river flow measured at Fernhill bridge was lower than 2,400 L/s for the past 20 years. For simplicity, the last week of February (week 9) has 8 days in leap years. Data obtained from the Hawke’s Bay Regional Council.
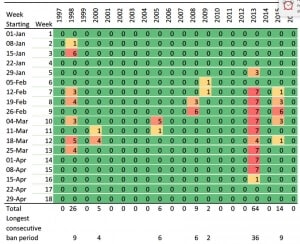
Table 2. Number of days per week in the period of January 1 to April 30 when the Ngaruroro river flow measured at Fernhill bridge was lower than 4,200 L/s for the past 20 years. For simplicity, the last week of February (week 9) has 8 days in leap years. Data obtained from the Hawke’s Bay Regional Council.
Figure 1. Ten-year average effective precipitation (dotted line) and vineyard evapotranspiration for Hawke’s Bay. Potential evapotranspiration (ETo) and precipitation data were retrieved from Roy’s Hill weather station for the period of 2005-2015. Crop evapotranspiration (ETc) was calculated from ETo and crop coefficients as described in Williams (2014). Effective precipitation was calculated as described by Brouwer and Heibloem (1986).
 Water moves from the soil through the roots, trunk, stems and leaves to the atmosphere, pulled by the suction effect of leaf transpiration. In decreasing soil moisture and high vapour pressure deficit, the suction force may be such that it breaks the water columns inside the xylem vessels, originating air bubbles that temporarily block the vessels. This process is called cavitation and decreases the hydraulic conductivity of the xylem. The increasing number and size of these bubbles can interrupt completely the xylem conduit, blocking its function: this phase is called embolism formation (Sperry, 1995).
Water moves from the soil through the roots, trunk, stems and leaves to the atmosphere, pulled by the suction effect of leaf transpiration. In decreasing soil moisture and high vapour pressure deficit, the suction force may be such that it breaks the water columns inside the xylem vessels, originating air bubbles that temporarily block the vessels. This process is called cavitation and decreases the hydraulic conductivity of the xylem. The increasing number and size of these bubbles can interrupt completely the xylem conduit, blocking its function: this phase is called embolism formation (Sperry, 1995).
In grapevine, embolism starts at moderate drought stress conditions when stem water potential is between -0.8 and – 1.2 MPa, (Lovisolo & Tramontini, 2010; Martorell et aI., 2015). There is a diurnal cycle of embolism formation and repair (Zufferey et aI., 2011) but vessels that have once embolised, are more prone to embolise again, opposing less resistance to new cavitation events (Stiller & Sperry, 2002). This is called “cavitation fatigue” and contributes to a loss of xylem function with repeated drought events.
Embolism repair is achieved by secretion of solutes (low molecular weight sugars) into embolised xylem by adjacent parenchyma and/or phloem thereby lowering osmotic potential inducing water flow and positive pressure build-up in the conduits (Nardini et al., 2011). Embolism repair can therefore potentially divert carbohydrates from other possible allocations within the plant, representing an additional cost, and decreasing production efficiency.
Loss of xylem function has an impact on stomata conductance and therefore indirectly affects photosynthesis potentially leading to carbon starvation. Carbon starvation affects productivity and can lead to plant decline and ultimately to plant death.
Depending upon the phenological stage at which water deficit occurs, water stress has a wide range of effects on grapevine growth, development and physiology. Water deficit during budbreak is not common in New Zealand vineyards but it has been reported to reduce vegetative growth and cause poor and uneven budbreak in South Africa and Washington State (Van Zyl 1984, Wample, 1997). Water availability influences canopy development and therefore sets an upper limit to the leaf area available for fruit ripening and replenishment of reserves. Shoot growth and leaf expansion are very sensitive to water availability and decrease linearly with leaf water potential (Y). When leaf water potential reaches -1 to -1.2 MPa, shoot elongation stops completely (Schultz & Matthews, 1988). Water deficit during pollination and early berry growth will cause embryo abortion, decreasing the number of seeds per berry, which will irreversibly limit berry size. Water deficit during the period between flowering and lag phase affects both cell division and cell enlargement in the developing berry, thus decreasing berry size (Matthews and Anderson 1989). The size of post-véraison berries is less impacted by water stress but ripening berries also shrink due to dehydration under severe water deficit leading to a concentration effect on berry solutes (Keller et al., 2006; Ojeda et al., 2001; Salón et al., 2005).
Vines respond to drying soil by closing the stomata to prevent excessive de-hydration. Communication between drying roots and shoot is achieved through chemical and hydraulic signalling. Abscisic acid (ABA) is produced in drying roots and transported to aerial parts to trigger stomatal closing to prevent excessive water loss. This causes the canopy temperatures to rise (no evaporative cooling from transpiration water) and hinders photosynthesis resulting in drastic reduction in vine carbon assimilation which will have a detrimental effect on fruit yield and composition (Chaves et al., 2010).
Persistent water stress depresses the fruitfulness of latent buds through a reduction of the number and size of inflorescence primordia (Alleweldt and Hofaecker 1975, Buttrose 1974), impacting the fruit yield of the current and subsequent seasons. Deficit late in the growing season (i.e., after véraison) restricts ripening and may jeopardise the realization of the following year’s yield potential by limiting the replenishment of storage reserves (Holzapfel et al., 2010; Zufferey et al., 2012).
Within the berry tissues, the flesh (mesocarp) is the most affected by water stress (Roby and Matthews, 2004). Smaller berries have higher surface to volume ratio and a higher proportion of skin:juice and seed:juice. Compounds that accumulate in skins and seeds such as anthocyannins and tannins will increase in fruit from drought stressed vines indirectly because of smaller berry size and directly because root derived ABA up-regulates genes encoding for enzymes of the phenyl-propanoid and flavonoid pathways responsible for the production of these compounds (Koyama et al., 2010). Severe water stress, however, especially before véraison, can lead to uneven ripening with some berries never changing colour. Drought stress may also increase the susceptibility to flavonol haze in the resulting wines via increased fruit exposure to UV light in less dense canopies.
Mild water stress increases the amount of volatile thiol precursors, independently of the influence of water status on berry size while more severe water stress decreases aroma potential (Peyrot des Gachons et al., 2004). Better fruit exposure will increase its temperature and sink activity. Water deficit early during berry development limits both tartaric and malic acid synthesis (Esteban et al., 1999). Higher fruit exposure results in faster post-véraison malate and methoxypyrazine degradation resulting lower concentrations of these compounds at harvest.
Although mild or moderate water deficit may increase sugar accumulation, limit berry size, and improve fruit composition by restricting shoot growth or by reducing canopy density (Ojeda et al., 2002; Shellie, 2014; van Leeuwen et al., 2004), severe stress can delay berry development and ripening because of a reduction in photosynthesis or even leaf abscission (Romero et al., 2010, 2013; Williams and Matthews, 1990; Williams et al., 1994).
We expect to have reductions in fruit yield, particularly when irrigation bans start early in the season. Drought will also curtail uptake of nutrients dissolved in the soil solution and result in nutrient deficiencies. Drought induced nitrogen deficiency will result in higher levels of phenolic compounds and lower levels of thiol precursors and may lead to stuck or sluggish fermentations.
The million-dollar question is: Can vines growing in Hawke’s Bay’s free draining soils survive long irrigation-ban periods? Will they recover unscarred upon re-watering? Another season like 2012-13 with the proposed low flow limit of 4,200L/s could easily result in unsustainable winegrape production in the affected areas. It is clear that there will be increasing incidences of water deficit at critical growth periods of vines in vineyards of the Ngaruroro catchment. It is also clear that there will be significant economic impacts with such events in terms of yield and fruit quality. What is unclear are the strategies to address such events.
Literature cited
Allen, R. G., Pereira, L. S., Raes, D., & Smith, M. 1998. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. FAO, Rome, 300(9), D05109.
Alleweldt, G., and W. Hofaecker. 1975. Influence of environmental factors on sprouting, flowering, fruitfulness and shoot growth in vines. Vitis 14:103-115.
Brouwer, C., & Heibloem, M. 1986. Irrigation water management: irrigation water needs (Vol. 3). Rome: FAO.
Buttrose, M.S. 1974. Fruitfulness in grapevines: Effects of water stress. Vitis 12:299-305
Chaves, M. M., Zarrouk, O., Francisco, R., Costa, J. M., Santos, T., Regalado, A. P., . . . Lopes, C. M. 2010. Grapevine under deficit irrigation: hints from physiological and molecular data. Annals of Botany, 105(5), 661-676.
Esteban, M. A., Villanueva, M. J., & Lissarrague, J. (1999). Effect of irrigation on changes in berry composition of Tempranillo during maturation. Sugars, organic acids, and mineral elements. American Journal of Enology and Viticulture, 50(4), 418-434.
Harding, X. (2016). Water update – an email to the Minister. Hawke’s Bay Wine, 34, 6-6.
Holzapfel, B.P., Smith, J.P., Field, S.K., Hardie, W.J. 2010. Dynamics of carbohydrate reserves in cultivated grapevines. Hortic Rev 37:143–211.
Keller, M., Smith, J.P., Bondada, B.R. 2006. Ripening grape berries remain hydraulically connected to the shoot. J Exp Bot. 57: 2577–2587.
Koyama, K., Sadamatsu, K., & Goto-yamamoto, N. (2010). Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Functional & Integrative Genomics, 10(3), 367-381.
Lovisolo, C., & Tramontini, S. (2010). Methods for assessment of hydraulic conductance and embolism extent in grapevine organs Methodologies and Results in Grapevine Research (pp. 71-85): Springer.
Martorell, S., Medrano, H., Tomàs, M., Escalona, J. M., Flexas, J., & Diaz‐Espejo, A. (2015). Plasticity of vulnerability to leaf hydraulic dysfunction during acclimation to drought in grapevines: an osmotic‐mediated process. Physiologia Plantarum, 153(3), 381-391. Matthews, M.A., and M.M. Anderson. 1989. Reproductive development in grape (Vitis vinifera L.): Responses to seasonal water deficits. Am. J. Enol. Vitic. 40:52-60.
Nardini, A., Lo Gullo, M. A., & Salleo, S. (2011). Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Science, 180(4), 604-611.
Ojeda, H., Andary, C., Kraeva, E,, Carbonneau, A., Deloire, A. 2002. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv Shiraz. Am J Enol Vitic. 53: 261–267.
Ojeda, H., Deloire, A., Carbonneau, A. 2001. Influence of water deficits on grape berry growth. Vitis 40: 141–145.
Peyrot des Gachons, C., Leeuwen, C. V., Tominaga, T., Soyer, J. P., Gaudillère, J. P., & Dubourdieu, D. (2005). Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. Journal of the Science of Food and Agriculture, 85(1), 73-85.
Roby, G., & Matthews, M. A. (2004). Relative proportions of seed, skin and flesh, in ripe berries from Cabernet Sauvignon grapevines grown in a vineyard either well irrigated or under water deficit. Australian Journal of Grape and Wine Research, 10(1), 74-82.
Romero, P., Fernández-Fernández, J.I., Martinez-Cutillas, A. 2010. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am J Enol Vitic. 61: 300–312.
Romero, P., Gil-Muñoz, R., del Amor, F.M., Valdés, E., Fernández, J.I., Martinez-Cutillas, A. 2013. Regulated deficit irrigation based upon optimum water status improves phenolic composition in Monastrell grapes and wines. Agric Water Manage. 121: 85–101.
Salón, J.L., Chirivella, C., Castel, J.R. 2005. Response of cv Bobal to timing of deficit irrigation in Requena, Spain: water relations, yield, and wine quality. Am J Enol Vitic. 56: 1–8.
Schultz, H., & Matthews, M. A. 1988. Vegetative growth distribution during water deficits in Vitis vinifera L. Functional Plant Biology, 15(5), 641-656.
Shellie, K. C. 2014. Water productivity, yield, and berry composition in sustained versus regulated deficit irrigation of Merlot grapevines. Am J Enol Vitic. 65: 197–205.
Sperry, J. S. (1995). Limitations on stem water transport and their consequences. Plant stems: physiology and functional morphology, 105-124.
Stiller, V., & Sperry, J. S. (2002). Cavitation fatigue and its reversal in sunflower (Helianthus annuus L.). Journal of Experimental Botany, 53(371), 1155-1161.
van Leeuwen, C., Friant, P., Choné, X., Tregoat, O., Koundouras, S., Dubourdieu, D. 2004. Influence of climate, soil, and cultivar on terroir. Am J Enol Vitic. 55: 207–217.
Van Zyl, J.L. 1984. Response of Colombar grapevines to irrigation as regards quality aspects and growth. S. Afr. J. Enol. Vitic. 5:19-28.
Wample, R.L. 1997. Important issues in vineyard irrigation. Good Fruit Grower 48:15-22, 39.
Williams, L. E. 2014. Determination of evapotranspiration and crop coefficients for a Chardonnay vineyard located in a cool climate. Am J Enol Vitic., 65, 159-169.
Williams, L.E., Matthews, M.A. 1990. Grapevine. In: Stewart BA, Nielson NR, eds. Irrigation of Agricultural Crops, Agronomy Monograph No 30. Madison, WI: 1019–1055.
Zufferey, V., Cochard, H., Ameglio, T., Spring, J.-L., & Viret, O. (2011). Diurnal cycles of embolism formation and repair in petioles of grapevine (Vitis vinifera cv. Chasselas). Journal of Experimental Botany, 62, 3885-3894.
Zufferey, V., Murisier, F., Vivin, P., Belcher, S., Lorenzini, F., Spring, J.-L., & Viret, O. 2015. Carbohydrate reserves in grapevine (Vitis vinifera L.’Chasselas’): the influence of the leaf to fruit ratio. Vitis 51(3), 103.
Acknowledgements: The author would like to thank the Hawke’s Bay Regional Council, in particular Vicky Bloomer for providing the data on Ngaruroro river flow levels.